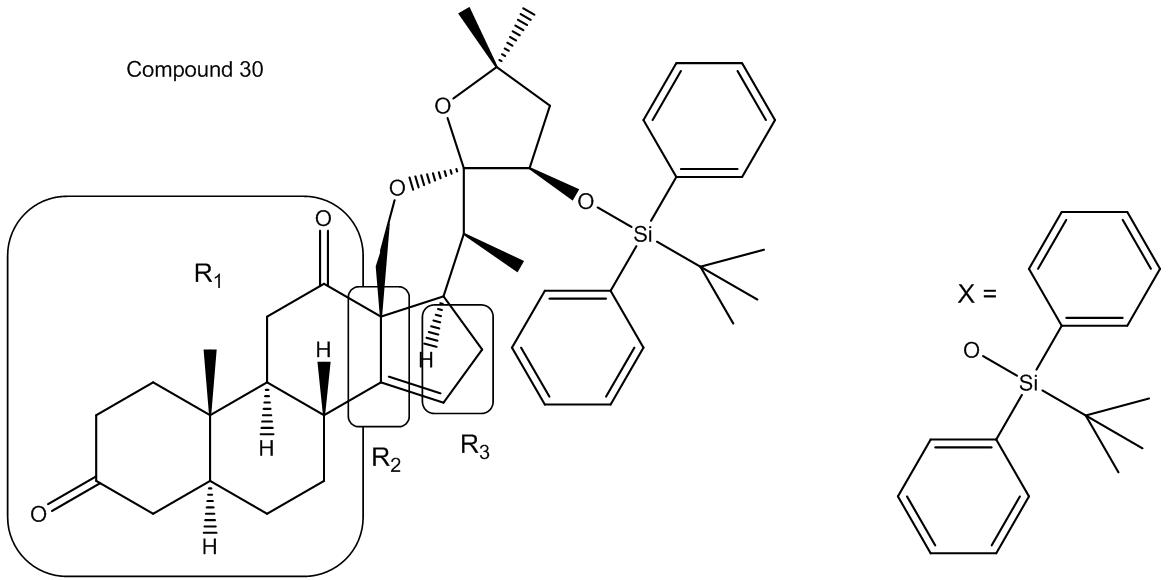
Provide a brief explanation as to why spiroketal 5 is thermodynamically more stable than its epimeric 30.
The more thermodynamically stable product among two possible anomers can be determined when the two main factors contributing to stability, steric interactions and the anomeric effect, are reinforcing in their effect.1 In the case of the spiroketal anomer in Compound 5 and the spiroketal in Compound 30 this is the case. As can be seen from the structure of the full compound shown below, both the structures of R2/R3 and X are bulky, and therefore produce highly unfavorable steric interactions when in the structure of compound 30. In addition, the structure of compound 5 is preferred due to the anomeric effect. It has been shown that in equilibrium between two anomers the one that has a substituent with lone pairs axial in relation a heteroatom on the six-membered ring is the preferred anomer.2 In the case of compounds 30 and 5 the six-membered ring is locked in the chair structure shown due to the nature of the “R” groups attached to the ring (because they are rings and the bonds cannot handle the strain of being axial and therefore opposite within their ring), the compound that has the heteroatom with lone pairs (in our case, oxygen) axial with respect to the oxygen in the ring is compound 5.
1Benito, M. J.; Rubio, E.; Gómez-Garcia, M.; Mellet, C. O.; Fernández, J. M. Tetrahedron 2004, 60, 5899-5906.
2Potuzak, J. S.; Moilanen, S. B.; Tan, D. S. J. Am. Chem. Soc. 2006, 127, 13796-13797.